 🖨️ Print post
🖨️ Print post
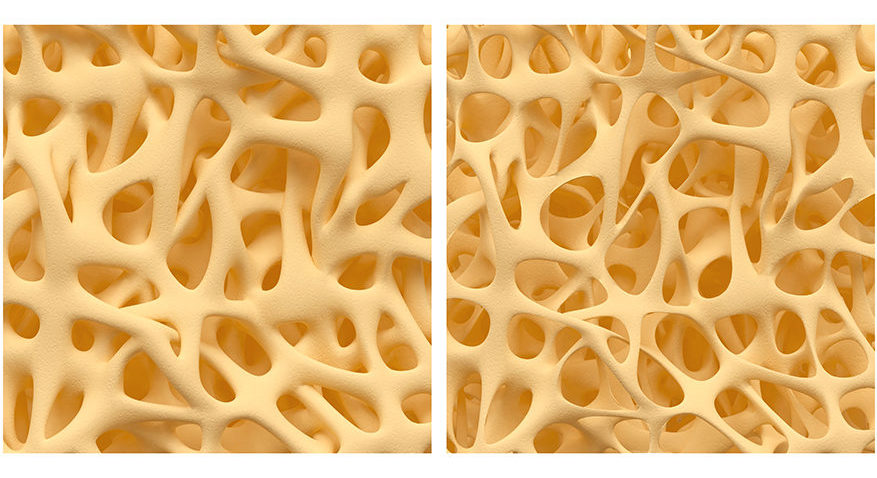
On the left is normal bone structure, and on the right is what would be described as osteoporosis.
Table of Contents
- Introduction
- Vitamin A, Bone Mineral Density, and Hip Fracture: The Epidemiological Evidence
- Synthetic Versus Natural: Does it Matter?
- Vitamin D: The Missing Link
- Vitamin D Protects Against the Toxicity of Vitamin A
- Vitamin D Requirements in the Vitamin D Winter
- Low Vitamin D Levels in Epidemiological Studies of Vitamin A and Osteoporosis
- Steroid Hormone Deficiency Mimics Vitamin D Deficiency
- Research Paradigms Must Change to Consider the Relationships Between Vitamins
- Getting Technical with Vitamins A and D: How They Interact to Regulate Bone Metabolism
- How Much Vitamin A is Too Much? The Wrong Question to Ask
- Towards a New Understanding of Vitamin Toxicity: Balance versus Amount
- Vitamin A is Still a Vitamin
- Carotenes: Neither Fully Adequate nor Fully Safe
- From Vitamins Back to Foods
- Appendix 1: Vitamins A and D Help Regulate Bone Remodeling
- Appendix 2: Does Vitamin A “Interfere” With Vitamin D?
- References
One of the many commonalities that Dr. Weston A. Price observed among the diets of the so-called “primitive” populations whom he described in Nutrition and Physical Degeneration, to which he attributed their resistance to dental caries and their superb skeletal structure, was a richness in the fat-soluble vitamins, including vitamin A. In fact, Dr. Price noted that indigenous diets were “at least ten times” higher in the fat-soluble vitamins than was the American diet, even assuming Americans were meeting the official recommendations of the day.1
Yet in recent years, some researchers have hypothesized that the osteoporosis seen among the elderly in modern civilizations, which manifests itself as reduced bone density and increased risk of fracture,2 is attributable to an excess of vitamin A. Many attendees of the Weston A. Price Foundation’s recent Wise Traditions 2005 conference were surprised and confused to hear Dr. Noel Solomons, director of the Guatemala-based CeSSIAM International Nutrition Foundation, a heroic program to improve vitamin A nutrition in third world countries, recommend a mere 800 international units (IU) per day of preformed vitamin A from animal foods – scientifically called “retinol” – and warn that, based on recent findings from the Nurses’ Health Study, intakes as low as 1500 IU per day are harmful to skeletal health.3
More recently, Dr. John Cannel, president of the Vitamin D Council and also a speaker at the Weston A Price Foundation’s 2005 conference, recommended in a newsletter that vitamin D supplements not contain any preformed vitamin A because it interferes with the function of vitamin D, and warned that “if you just have to take” cod liver oil, “don’t take more than a teaspoon a day.” Dr. Cannel suggested ß-carotene, a precursor to vitamin A (retinol) that is found in plant foods, is a safe alternative to the preformed retinol found in cod liver oil.4
Although the vitamin supplement industry claims that the research is conflicting and inconclusive,5 there is actually an impressive body of evidence suggesting that, in certain circumstances, an “excess” of vitamin A is contributing to an increased risk of osteoporosis in certain populations even at relatively low levels.
At first glance, the research seems to stand in stark contrast to Price’s consistent observation of very high levels of vitamin A in native diets accompanying the superior skeletal health of those same groups. A more careful consideration of the research suggests, however, that what is at issue is not an excess of vitamin A, but an imbalance between vitamin A and other nutrients in the diet, especially vitamin D. Human and animal evidence strongly suggests that vitamin A can only exert harm against the backdrop of vitamin D deficiency, that sufficient levels of vitamin A are even higher than once thought, and that supplementing with carotenes is neither an adequate nor a safe way to achieve these optimal levels – all of which are consistent with and supportive of Price’s timeless findings.
Vitamin A, Bone Mineral Density, and Hip Fracture: The Epidemiological Evidence
Just twelve years after vitamin A was discovered as a constituent of cod liver oil and butterfat, a team of researchers led by Takahashi established in 1925 that it produced toxic symptoms (now called “hypervitaminosis A”) in rats when fed as a natural fish oil concentrate at 10,000 times the required amount. In 1933, these researchers showed that this vitamin A-rich concentrate was toxic to bone in extreme doses, resulting in spontaneous fractures, and in 1945 Moore and Wang confirmed that these effects were attributable to vitamin A by inducing them with purified retinyl acetate, which is, like retinol, a type of preformed vitamin A. Researchers have since reported skeletal lesions in response to extreme doses of vitamin A in dogs, pigs, rabbits and chickens. In humans, an increase in blood levels of calcium (caused by the leaching of calcium from bone), bone pain, and other bone-related symptoms sometimes accompany hypervitaminosis A.6
Yet it is not such toxic, wildly excessive doses of vitamin A to which modern researchers are attributing osteoporosis, but normal, even relatively low, non-toxic intakes of vitamin A common in modern societies – intakes far below those of the tribesmen and villagers whom Weston Price found to have superb skeletal health.
Summary of the Epidemiological Evidence
In 1998, a group of Swedish researchers led by Hakan Melhus observed that hip fracture rates vary seven-fold across Europe and are highest in Northern Europe, Sweden, and Norway – where northern latitudes preclude the UV-induced synthesis of vitamin D in human skin for much of the year, and where vitamin D intakes are frequently well below the paltry recommended minimums,6 which they failed to note – and where intake of preformed retinol is six-fold higher than elsewhere in Europe, which they noted duly. The group studied women from Uppsala, a county of Sweden, and published the first study on vitamin A intake, bone mineral density (BMD) and hip fracture risk that distinguished between preformed retinol, found in animal foods, and its precursors, carotenes, found in plant foods. They found that, compared to an intake of retinol below 1,700 IU per day, intakes of retinol exceeding 5,000 IU per day were associated with a six percent decrease in total body BMD, a 10 percent decrease in BMD at the site of the hip, and a doubling of the risk of hip fracture.7
Several groups of researchers have published conflicting studies since 1998, using different methods of estimating vitamin A intake. Among them:
- Ballew and others published a study in 2001 finding no relationship between blood levels of vitamin A and bone mineral density in Americans in the Third National Health and Nutrition Examination Survey (NHANES-III).8
- In 2002, Feskanich and others published findings from among American nurses who participated in the Nurses’ Health Study showing that intakes of preformed retinol, but not carotenes, were associated with the risk of hip fracture among postmenopausal women who were not taking hormone replacement therapy (HRT). An intake above 1,700 IU per day was associated with a somewhat inconsistent increased risk of fracture, while an intake above 6,700 IU per day was associated with a much sharper and more consistent increased risk of fracture.9
- The same year, Promislow and others reported a U-shaped curve between retinol intake and BMD among Southern Californian elderly women and men in the Rancho Bernardo study, in which an ideal intake of preformed retinol amounting to 2000-2800 IU per day was associated with the greatest BMD, while either an increase or decrease from this amount was associated with a lower BMD. The trend was more consistent and stronger among the postmenopausal women and less pronounced among men.10
- In 2003, a study by Michaelsson, Melhus, and others found a similar U-shaped curve for blood levels of vitamin A and the risk of fracture in elderly men of Uppsala, Sweden. Compared to the middle quintile, the highest quintile of retinol levels had a 64 percent increased risk of any fracture and a 147 percent increased risk of hip fracture. Compared to the same middle quintile, men with retinol levels above 3.5 micromoles per liter (µM, which is a measure of a specific number of vitamin A molecules per liter of blood) had seven times as many fractures. The researchers concluded that risk is primarily associated with levels above 3 µM. Although to a lesser extent, risk of fracture increased in the lower quintiles of serum retinol as well.11
- In 2004, a team led by Opotowsky published a study of American women that established a much cleaner U-shaped curve associating blood levels of vitamin A with hip fracture risk than the curve established by Michaelsson’s team. The middle quintile, which grouped those with blood levels of vitamin A between 1.9 and 2.13 µM, had the lowest risk of fracture. Compared to this quintile, the lowest and highest quintiles both carried approximately double the risk of fracture.12
- The same year, Lim and others published a study drawing from data representing over 41,000 postmenopausal women from the Iowa Women’s Health Study, which found no association between the dietary intake of preformed retinol and the risk of hip fracture. Those who used vitamin A supplements, as a group, had a 17 percent increased risk of hip fracture compared to those who did not, but there was no relationship between the amount of vitamin A taken and the risk of hip fracture.13
- Barker and others published a study in 2005, this one also failing to find a positive association between vitamin A and fracture risk. In this study, researchers measured the blood levels of vitamin A among British women over the age of 75. Over the following four years, the researchers actually found a 15 percent decrease in the risk of osteoporotic fracture among the highest quintile of vitamin A levels. About 40 percent of the subjects were using cod liver oil or a multivitamin, but no information was provided to differentiate between the two. This was the first of these studies to mention cod liver oil specifically as a source of vitamin A supplementation.14
Stronger Studies Show an Association
Although those of us who would like to feel reassured of the safety and benefit of a high vitamin A intake without giving the subject careful thought may be tempted to brush off this research as conflicting and therefore inconclusive, studies that do not find an association between vitamin A and BMD or fracture risk generally suffer from some inadequacy, being bettered by their counterparts that do find an association.
For example, the Ballew study analyzed the data in such a way as to reveal a linear relationship between blood levels of vitamin A and BMD, where an increase from one vitamin A level to another would consistently yield a decrease of BMD. It did not, however, look for a U-shaped curve, where both a decrease or an increase from an ideal level of vitamin A could lower BMD.8 When the Opotowski team analyzed its data in the same way, it found a relative risk of 1.0 for blood levels of vitamin A, meaning that fracture risk appeared to be distributed perfectly evenly among all vitamin A levels, yielding no relationship. Yet when the Opotowski team analyzed the same data looking for a U-shaped curve, it found high and low serum vitamin A levels both to carry double the risk of fracture as did optimal levels – something the Ballew team couldn’t have found because of the way the group analyzed the data.12
Although the Lim team found no relationship between dietary intake of preformed retinol and fracture risk, it only used one one-week food frequency questionnaire (FFQ),13 whereas the 1998 Melhus study used four one-week FFQs,7 and the Nurses’ Health Study used five one-week FFQs. In fact, when the Feskanich group analyzed the Nurses’ Health Study using only the data from the first FFQ, the association between the fifth and first quintiles of retinol intake fell from a statistically significant (meaning consistent and unlikely to be due to chance) 89 percent increased risk to a non-statistically significant (meaning inconsistent, with a high probability of being due to chance) 17 percent increased risk.9 This suggests that one one-week FFQ does not measure retinol intake with sufficient accuracy to detect an association, and that, had the intake data more accurately estimated real intake of retinol, the Lim team might have found such an association.
Synthetic Versus Natural: Does it Matter?
So far, things are looking bleak for vitamin A. To those of us familiar with the long-established findings of Dr. Price that associate diets very rich in vitamin A with vibrant skeletal health, the modern research that shows much lower amounts of vitamin A to contribute to osteoporosis paints a picture of vitamin A that appears not only bleak, but also confusing. Many of us may be tempted to argue that it is not natural vitamin A-rich food that contributes to osteoporosis, but the synthetic preparations of vitamin A found in multivitamins and used to fortify foods like breakfast cereals, margarine, and low-fat milk. Yet under closer scrutiny, this familiar intellectual weapon in our arsenal proves impotent, and the answer must lie elsewhere.
At first glance, two pieces of evidence seem to suggest synthetic vitamin A is exclusively the culprit. Indeed, a full 94 percent of the variance in retinol intakes between the highest and lowest quintiles in the Nurses’ Health Study was attributable to supplemental retinol, while only six percent of the variance between these quintiles was attributable to food retinol.9 Seemingly even more powerful, the Rancho Bernardo study found that those taking supplemental vitamin A had a dose-dependent decrease in bone mineral density (BMD) as they consumed more retinol, while those obtaining retinol from food alone had a dose-dependent increase in BMD as they consumed more retinol.10
Close examination shows, as the researchers noted, that the apparent difference between supplement users and supplement non-users in the Rancho Bernardo study is an illusion created by the difference in total vitamin A intakes between the two groups. Those who obtained their retinol from food alone had low intakes of retinol, while those who obtained their retinol from both supplements and food together had high intakes of retinol, with only a small portion of overlap between the intakes of the two groups.10
Figure 1

Reproduced from J Bone Miner Res 2002;17:1349-1358 with permission of the American Society for Bone and Mineral Research. The lines punctuated by squares (left) represent subjects who obtained retinol from food only; the solid lines (right) represent subjects who obtained retinol from food and supplements; the dotted curves represent the two combined. As a line moves upward, bone mineral density increases. As a line moves rightward, retinol intake increases. The “X” where the two lines cross represents the points for which the two groups were consuming the same amount of retinol. Even when consuming the same amounts of retinol, bone mineral density increases with increasing retinol intake for those not taking supplements and decreases with increasing retinol intake for those taking supplements. However, if one imagines the square-punctuated line shifted to the left to account for supplemental retinol being utilized at a higher rate, a U-shaped curve emerges for all retinol intakes.
Still one puzzle remains: In the graph in Figure 1, the line on the left, representing the BMD of subjects who obtained retinol from food alone, crosses the line on the right, which represents those who obtained retinol from supplements, yet even in this overlap, food retinol remains protective, while supplemental retinol remains destructive – something the researchers didn’t explain. The answer is likely found in the fact that fat-soluble retinol found in food and some forms of supplements results in lower peak plasma values (levels in the blood), lower liver stores, and higher fecal loss than the water-soluble, emulsified, and solidified forms of vitamin A found in most supplements.15 Additionally, although the type of vitamin A found in supplements, all-trans retinol, is the most common type found in food, foods also contain a variety of other forms of vitamin A, all of which have lower levels of activity than all-trans retinol.16
In other words, a given amount of retinol from food is effectively a lower dose of retinol than the same amount from most forms of supplements. If we imagine the line to the left in Figure 1 to be shifted leftward to compensate for this difference in availability, the picture that emerges, then, is a clean U-shaped curve similar to that found by the Opotowsky team with blood levels of vitamin A. The implication of this important fact is that larger amounts of natural vitamin A will have the same effect on bone mineral density as smaller amounts of supplemental vitamin A.
The results of the Nurses’ Health Study are particularly damning to the idea that it is synthetic, but not natural, vitamin A that contributes to osteoporosis. The Feskanich group reported the proportion of various dietary sources of vitamin A in this study in great detail and differentiated between supplemental and food retinol with impressive rigor. When the figures were adjusted for age only, the highest intake of retinol from food and supplements combined carried a 27 percent increased risk over the lowest quintile of intake. The researchers performed a second analysis that excluded all persons who consumed any form of supplemental vitamin A. For those obtaining retinol from food alone, the highest quintile of intake carried a 67 percent increase in risk!9
The researchers then performed a multivariate analysis, which adjusted for intake of vitamins D and K, protein, calcium, alcohol, and caffeine, as well as smoking, use of certain drugs and hormone replacement therapy, body mass index and other factors in addition to age. In this analysis, intake of retinol from food and supplements combined yielded an 89 percent increased risk among the highest quintile of intake compared to the lowest, while those obtaining retinol from food alone had a somewhat lower 67 percent increased risk in the highest quintile compared to the lowest. The authors duly noted that even those not using supplemental vitamin A were obtaining vitamin A from fortified foods in addition to naturally occurring vitamin A, and performed a third analysis for that reason: those who consumed liver at least once a week had a 69 percent increased risk of fracture compared to those who never ate liver.9
Although there are a variety of reasons to obtain vitamin A from foods rather than supplements, avoiding the risks of so-called “excessive” intakes of vitamin A with respect to osteoporosis is not one of them. The research clearly suggests that the amount of vitamin A is the operative factor rather than the form of vitamin A.
At this point, the evidence against vitamin A appears on the surface to be inescapably incriminating. There is, however, much more to the story.
Vitamin D: The Missing Link
Most studies investigating the link between vitamin A and osteoporosis view vitamin A “excess” in a vacuum, as if the only factor determining what constitutes an excess of vitamin A for a given body weight is the amount of vitamin A itself. Indeed, the toxicity of vitamins in general is often determined by using large doses of a single vitamin. This may help us attribute toxic effects to a particular vitamin, but it also precludes our understanding which effects are due to the absolute amount of that vitamin, and which effects are due to an imbalance between that vitamin and other vitamins.
Vitamin D Protects Against the Toxicity of Vitamin A
Research that examines the feeding of high doses of more than one vitamin simultaneously reveals that toxicity is dependent on reactions between different nutrients. For example, studies in rats, turkeys, and chickens have demonstrated that vitamin A both decreases the toxicity of and increases the dietary need for vitamin D, while vitamin D both reduces the toxicity of and increases the dietary need for vitamin A.6
In 2003, Myhre and other researchers examined all 291 cases of hypervitaminosis A in humans reported in the medical literature between 1944 and 2000. Of these, the Myhre team identified 81 reports that provided information about the patient’s vitamin D supplementation, and found that concomitant supplementation with vitamin D radically increased the dose of vitamin A needed to cause toxicity. Unfortunately, the researchers only mentioned whether vitamin D was supplemented at all and did not discuss the specific amount of vitamin D being supplemented. Nevertheless, they found that the median dose reported for vitamin A toxicity was over 2,300 IU per kilogram (kg) of body weight per day higher when vitamin D was also supplemented. For a hypothetical 75-kg person representing the median, vitamin D supplementation would have allowed an additional 175,000 IU per day (the amount in five tablespoons of high-vitamin cod liver oil) before toxicity symptoms were likely to be reported!15
Vitamin D Requirements in the Vitamin D Winter
It is difficult to resist the observation that Scandinavian countries, which have the highest fracture rates in Europe, not only have higher average intakes of retinol but also exist at far northern latitudes, where “vitamin D winters” – periods of time during which vitamin D cannot be produced by the action of sunlight upon the skin – are longer and less vitamin D is available from the sun in the months during which it is available at all.
The ideal way to obtain vitamin D is by exposure to sunlight. Sunshine in the ultraviolet-B (UV-B) spectrum strikes the skin, converting a cholesterol precursor called 7-dehydrocholesterol into vitamin D3, which is also called cholecalciferol, as well as a variety of similar chemicals, including the activated form of vitamin D, calcitriol. When atmospheric conditions are ideal and skies are clear, 30 minutes of whole-body exposure of pale skin to sunlight without clothing or sunscreen can result in the synthesis of between 10,000 and 20,000 IU of vitamin D. These quantities of vitamin D are large, and therefore capable of supplying the body’s full needs. At the same time, the body has two mechanisms to prevent an excess of vitamin D from developing: first, further irradiation converts excess vitamin D in the skin to a variety of inactive metabolites; second, the pigment melanin begins to accumulate in skin tissues after the first exposure of the season, which decreases the production of vitamin D.17
The availability of UV-B rays, however, depends on the angle at which sunshine strikes the earth, making vitamin D synthesis impossible for most people at most latitudes during parts of the year called the “vitamin D winter.” Outside the vitamin D winter, sufficient UV-B rays for full vitamin D synthesis do not suddenly become available: the window of time during each day in which vitamin D synthesis can occur gradually expands as the season progresses, as does the amount of UV-B radiation available within that window. In 1988, Webb and other researchers established that some degree of vitamin D winter occurs above 34 degrees latitude, and that in Boston, at 42.2 degrees north, the vitamin D winter extends for four months from November through February, while in Edmonton at 52 degrees north, it extends across six months from October through March.18
In 2005, Engelsen and colleagues published a study that suggests the Webb team may have overestimated the true vitamin D winter for clear skies in Boston, probably by mistaking scattered clouds, which are almost always present even when the sky appears clear, for cloudlessness. Using a more precise model, the Engelsen team accounted for the following factors: natural variations in the density of the ozone layer can cause the length of the vitamin D winter to increase or decrease by up to two months; clouds can eliminate up to 99% of UV-B radiation; aerosols and the presence of buildings decrease exposure to UV-B; finally, increased altitude and reflective surfaces such as snow increase exposure to UV-B. While the Engelsen team found that the vitamin D winter would be shorter than estimated by the Webb team under truly “clear” skies, it also found that the aforementioned factors can extend the vitamin D winter so dramatically that such a winter can sometimes occur even at the equator.19
The county of Uppsala, Sweden, where Michaelsson, Melhus, and others had associated retinol intake with reduced BMD and increased risk of fracture in 1998, 7 and likewise had associated serum retinol levels with the risk of fracture five years later,11 is located at the northern latitude of 59.97 degrees.20 Using an online model provided by the Engelsen team21 and assuming typical atmospheric conditions and complete cloudlessness – an idealized and rarely occurring phenomenon – I estimate that Uppsala’s vitamin D winter would extend for a minimum of about four months, from late October to late February. However, dense ozone and overcast skies could cause the vitamin D winter to extend for more than ten months from mid-July, to the end of May. The typical vitamin D winter probably lies somewhere between the two.
In order to maximize calcium absorption, blood levels of 25 (OH) D – the form of vitamin D that the body stores in its reserves – must be maintained at 30 ng/mL (nanograms per milliliter – a nanogram is a billionth of a gram), which, in the absence of UV-B light, would require roughly 2600 IU per day of vitamin D to maintain. However, fracture risk continues to decline at even higher levels of 25 (OH) D because people actually fall less often, suggesting that these higher vitamin D levels enhance neuromuscular coordination. Higher vitamin D levels also confer benefits that are unrelated to the skeletal system: serum levels as high as 46 ng/mL, for example, appear to maximize the body’s ability to regulate blood sugar. Dark-skinned agricultural workers in the tropics tend to have 25 (OH) D levels of about 60 ng/mL, suggesting that the optimal level of vitamin D is nearer to this figure.22
According to Dr. John Cannel of the Vitamin D Council, to maintain serum levels of 25 (OH) D at the suggested optimal range of 50 ng/mL during a vitamin D winter, one must consume 4000 IU of vitamin D per day.23 By contrast, the groups studied by Melhus and Michaelsson were consuming much less.
Low Vitamin D Levels in Epidemiological Studies of Vitamin A and Osteoporosis
Although Michaelsson and Melhus did not report the vitamin D intake of the Uppsala men and women whom they studied in either of their two reports associating vitamin A with osteoporosis, they authored another report on a different subject, studying women of Uppsala and the adjacent county, Vastmanland, in which they reported vitamin D intakes organized by quintile of calcium intake. The women in the lowest quintile of calcium intake consumed an average of only 97 IU of vitamin D per day, while the women in the highest quintile of calcium intake consumed an average of only 185 IU of vitamin D per day.24 Thus, at a latitude that spends the preponderance of the year covered under the dusk of a vitamin D winter, the residents of Uppsala are consuming between one twentieth and one fortieth of what is required to maintain optimal serum levels of vitamin D.
The majority of epidemiological studies investigating the hypothesized link between vitamin A and osteoporosis have not reported vitamin D levels. What little data we can glean from the few that have, however, suggests that the relative amounts of vitamins A and D may be more important than the amount of vitamin A alone.
In the Nurses’ Health Study, which found a positive association between retinol intake and fracture risk, intake of vitamin D increased as intake of retinol increased, but at a much lower rate. The net effect was that the retinol-to-vitamin D ratio increased from 9.7 in the lowest quintile of retinol intake, which had the lowest risk of fracture, to 19.34 in the highest quintile of retinol intake, which had the highest risk of fracture. The researchers found vitamin D intake to be protective, and multivariate analysis that adjusted for many variables including vitamin D intake caused the association with vitamin A to become much more pronounced and consistent.9
That the effect of vitamin A became more pronounced when vitamin D was controlled for shows that the net effect of a higher vitamin A intake depends not only on the amount of vitamin A consumed, but also on the amount of vitamin D consumed with it. Since vitamin D is produced in the skin by sunlight but only vitamin D consumed in the diet, and not blood levels, was reported, we can’t know for sure the true vitamin D status of the participants in the Nurses’ Health Study, but to the extent that they relied on dietary vitamin D, the evidence suggests that the increase in hip fractures may be due not simply to an increase in vitamin A intake itself, but to an increase in vitamin A considerably out of proportion to the paltry increases in the very low intakes of vitamin D.
By contrast, in the Barker study, which found a negative association between serum retinol levels and fracture risk, serum vitamin D increased as serum retinol increased, but at a higher rate. Unfortunately, the researchers made no differentiation between a multivitamin and cod liver oil for the 40 percent of study participants who took one or the other. Nevertheless, the explicit mention of cod liver oil as a source of vitamin A supplementation suggests that its use was substantial. Those not using a supplement had mean serum retinol levels of 1.95 µM and mean serum 25 (OH) D levels of 15.24 ng/mL, while those using a multivitamin or cod liver oil had mean serum retinol levels of 2.07 µM and mean serum 25 (OH) D levels of 18.88 ng/mL. This represents an increase in serum retinol levels of 6.15 percent, and an increase in serum vitamin D levels of 23.9 percent. In this study, the highest quartile of serum retinol carried a 15 percent decrease in fracture risk, while using a multivitamin or cod liver oil, which increased serum D levels at nearly four times the rate at which it increased serum retinol levels, carried an even greater 24 percent decreased risk of fracture.14
When taken together, these two studies demonstrate that a higher intake or blood level of vitamin A does not necessarily, by itself, lead to an increase or decrease in fracture risk; instead, an increase in vitamin A can be harmful if not accompanied by a sufficient increase in vitamin D, or healthful when accompanied by such an increase.
One study that does not support this view is the Lim group’s analysis of the Iowa Women’s Health Study. Although ratios of retinol intake to vitamin D intake doubled between the lowest and highest quintile, no association of any kind was found between vitamin A intake and fracture risk. While use of a vitamin A supplement decreased the ratio of retinol intake to vitamin D intake, it also resulted in a increased risk of fracture; since there was no relationship between the amount supplemented and the decrease of risk, however, the significance of the finding is questionable.13 As noted earlier, this study suffers from a major deficit: the researchers used only one FFQ, a practice which produced similarly null results in the Nurses’ Health Study, in contrast to the positive results produced from the Nurses’ Health Study when the same researchers used all five FFQs.
Just as the higher quality studies demonstrate a relationship between vitamin A and osteoporosis, the higher quality studies among those that report both vitamin A status and vitamin D status demonstrate that whether vitamin A is harmful or healthful depends on whether sufficient vitamin D is consumed with it.
Steroid Hormone Deficiency Mimics Vitamin D Deficiency
Finally, it should be reiterated that the Nurses’ Health Study found the association between vitamin A and fracture risk to be concentrated among postmenopausal women not using hormone replacement therapy (HRT). When the researchers performed a separate analysis of those using HRT and those not using HRT, there was no consistent relationship between retinol intake and fracture risk among those using HRT, while multivariate analysis yielded a statistically significant 252 percent increased risk in the highest quintile of vitamin A intake among women not using HRT .9
Estrogen and other sex steroids play some roles in the body that are similar to roles played by vitamin D. For example, estrogen is a primary inhibitor of bone resorption in both men and women,26 while both estrogens and androgens increase intestinal absorption and the retention of calcium,xii both of which are also roles played by vitamin D.6 Estrogen, testosterone, and other androgens also play roles in facilitating bone growth.25 It could be, then, that a decline in sex steroids aggravates the effect of vitamin D deficiency, bringing the total vitamin D-like activity to a low enough level that a higher intake of vitamin A begins to become harmful. It is enlightening that sex steroid deficiency appears to “turn on” the otherwise dormant association between vitamin A and fracture risk: this should give us pause to consider whether this same association can be “turned on” by vitamin D deficiency and “turned off” by vitamin D sufficiency in the same way – much like the flip of a light switch.
Research Paradigms Must Change to Consider the Relationships Between Vitamins
Although researchers in general have paid altogether too little attention to the balance between vitamins A and D when examining the relationship between vitamin A and osteoporosis, one researcher, Sara Johansson, a tutee of Hakan Melhus, has made this relationship a central part of her hypothesis. Johansson noted with Melhus in a 2001 paper that vitamin D intakes in Scandinavia are often deficient and sunlight is limited, concluding, “We hypothesize that the high intake of vitamin A in Scandinavia may aggravate further the effect of hypovitaminosis D on calcium absorption.”27
Similarly, Johansson concluded her 2004 PhD thesis by writing, “I hypothesize that the high intake of vitamin A in Scandinavia may further aggravate the effect of hypovitaminosis D on calcium absorption and, possibly, contribute to the high incidence of osteoporosis . . . It would also be interesting to see if the outcome of epidemiological studies would be different if, in addition to the level of vitamin A intake, consideration was taken to the vitamin D status of the individuals.” Unfortunately, even Johansson went on to repeat the error of those who, unlike her, see vitamin A “excess” as a phenomenon operating in a vacuum rather than a phenomenon dependent on balance with vitamin D, with these final words: “It has become clear that vitamins can not be considered magic pills that bring only benefit to health, and that the widespread misconception – ‘the more vitamins the better’ – is a fallacy.”6
Certainly, the statement taken by itself is important and true. Without doubt, there must be a point beyond which the fat-soluble vitamins become harmful rather than healthful, as do all substances. But to suggest that this is the most powerful lesson to be concluded from the research on vitamin A and osteoporosis is to suggest that vitamin A intakes in modern society are currently too high. Yet there are two other possibilities: first, high vitamin A intakes might be safe and beneficial when vitamin A is consumed in the proper ratio to vitamin D; second, a wide range of intakes and ratios between the two vitamins might be acceptable if sufficient levels of both are maintained.
Johansson’s own research into the interactions between vitamins A and D, and that conducted by other researchers, suggests that the last two possibilities are much more likely explanations than the first. In fact some animal experiments have shown that unthinkably massive doses of vitamin A are safe when accompanied by equally massive doses of vitamin D.
Before we review this fascinating research, let’s review some of the technical details of vitamin A’s role in bone metabolism, and how it interacts with vitamin D.
Getting Technical with Vitamins A and D: How They Interact to Regulate Bone Metabolism
An Introduction to Bone Anatomy and Metabolism
Bone is a living tissue comprised mostly (90-95 percent) of a collagen matrix, an assortment of other types of proteins, and deposited hydroxyapatite crystals, which are made primarily of calcium and phosphorus salts. Within bone are three types of cells: osteocytes, which burrow canals and blood vessels through bone to supply nutritive support; osteoclasts, which secrete acids and protein-digesting enzymes that dissolve bone; and osteoblasts, which support the growth of new bone by secreting the collagen-based matrix, which itself attracts the deposition of mineral salts. Precursors to osteoblasts and osteoclasts lie on the surface of bone, each of which develops into mature and active osteoblasts and osteoclasts, respectively, when directed to do so by certain signaling molecules. As osteoblasts secrete new bone matrix, some of these cells become trapped in their own matrix and develop into osteocytes.6
Bone resorption, performed by osteoclasts, and bone growth, performed by osteoblasts, are complimentary to one another, and together make up the process called bone remodeling, which allows bones to optimize their shape in response to environmental cues, to adjust to the occurrence and repair of injury, and to allow the body to tightly regulate calcium levels. During childhood and adolescence, the balance between the two favors bone growth, until peak bone mass is reached between the ages of 25 and 30. Ideally, the maintenance of evenly balanced bone remodeling persists after this point, but typically in older age an imbalance occurs in favor of bone resorption, which contributes to decreased bone mineral density (BMD) and therefore to osteoporosis.6
Osteoporosis is defined as “a skeletal disorder characterized by a reduction in bone mass with accompanying microarchitectural damage that increases bone fragility and the risk for fracture.”2 Low BMD itself can only account for 28 percent of fractures. Fracture risk is also determined by the mechanical quality and geometry of the joint. Another factor in fracture risk is so obvious that it may go overlooked: the propensity to fall.6 This might actually be, to some degree, nutritionally determined: as discussed above, one study associated high serum vitamin D levels with decreased likelihood to take a fall, interpreted by some to attribute a neuromuscular benefit to high levels of vitamin D.22
Bone Resorption: A Positive and Necessary Function Regulated by Vitamins A and D
The activated forms of vitamins A and D, retinoic acid and calcitriol respectively, are both hormones, which are signals that cause changes in cells by altering the expression of genes, or activating one or another enzyme within the cell that carries out some function. Retinoic acid activates bone resorption by increasing the number and activity of osteoclasts. It also decreases the growth of osteoblasts. The oft-cited and conventionally understood role of calcitriol is to inhibit bone resorption,6 but mice that have no vitamin D receptor have impaired bone resorption, indicating that calcitriol also plays a role in stimulating bone resorption.28
While bone resorption and bone growth are in a sense “antagonistic,” they really are complimentary processes. Osteoblasts and osteoclasts synergistically regulate each other. Although osteoclasts perform bone resorption, it is osteoblasts that initiate the process, by secreting signals that cause osteoclast precursor cells to develop into mature osteoclasts. As osteoclasts erode bone, various growth factors are released from the eroded bone that in turn stimulate the maturation of osteoblasts. As osteoblasts mature, they progressively make fewer osteoclast-activating signals and more osteoclast-inhibiting signals, so that bone resorption gradually slows and eventually comes to a stop just before bone growth begins.6
Vitamin A’s bone resorption-stimulating activity is vitally important to bone health. The Opotowski team, which found that low vitamin A levels had as great an effect lowering BMD as did high vitamin A levels, suggested that vitamin A deficiency may contribute to increased fracture risk by allowing bone matrix to grow faster than it can be mineralized.12 Indeed, although the net effect of vitamin A is to stimulate osteoclasts and slow the growth of osteoblasts, vitamin A also causes osteoblasts to secrete a variety of enzymes and other proteins that are important to bone mineralization, including osteocalcin, which is a protein that plays a direct role in attracting and binding calcium within the bone matrix.6 By slowing the growth of the matrix but increasing the rate at which it is mineralized, adequate vitamin A helps to ensure sufficient bone density.
Rickets, a vitamin D-deficiency disease that occurs in developing children, is marked by a massive increase in osteoid tissue (non-mineralized bone matrix), an increase in the number of osteoblasts, and an inability of osteoclasts to perform bone resorption. Rickets is accompanied by a dramatic expansion of a portion of the bone called the metaphyseal plate, and total bone volume can actually increase. The adult version of rickets, osteomalacia, a term also used to refer to the unregulated osteoid growth that occurs in childhood rickets, is also sometimes accompanied by a decrease in the number of osteoclast cells.28 Thus, bone resorption is a vitally important process that both vitamins A and D play a role in stimulating and regulating.
(See Appendix 1 for more details on the regulation of bone remodeling by vitamins A and D.)
Vitamins A and D Regulate the Intestinal Absorption of Calcium and Phosphorus
Vitamin D’s primary role in bone health is not to act directly on bone cells, but to increase the intestinal absorption of calcium. Mice that have been modified to not possess the vitamin D receptor (VDR) develop rickets and osteomalacia when fed a diet that is one percent calcium and 0.67 percent phosphorus. They have 30 times the osteoid tissue of controls, suffer a seven-fold decrease in bone stiffness, and experience a marked expansion of the metaphyseal plate, a primary sign of rickets. However, when the amount of calcium and phosphorus in the diet is doubled, mice with no VDR develop normally without any such changes.28
Vitamin D acts through the VDR to increase the expression of various proteins in the intestines that are involved in transporting calcium across the intestinal border and through intestinal cells into the blood, and by less-understood mechanisms also increases the absorption of phosphorus. When the calcium concentration of the diet is very high, however, calcium passively diffuses across the border of the intestines.29 Phosphorus, in turn, must be consumed in the proper proportion to calcium – which, in vitamin D-deficient rats is one to two30– which explains why a diet high in calcium and phosphorus would be able to bypass the effects of an absent VDR.
Still, we can not rule out an effect of vitamin D on calcium and phosphorus absorption even in these experiments, since the mice that lacked a VDR still consumed some vitamin D. In 2005, researchers identified a mammalian protein they termed 1,25D3-MARRS present in the outer membrane of intestinal cells that induces a rapid response to vitamin D, enhancing the transport of phosphorus31 and possibly calcium32 into the cell, showing that not all of vitamin D’s actions occur through the VDR.
In both the rat33, 34 and the human,27 vitamin A antagonizes the rise in serum calcium that is induced by vitamin D. Johansson and Melhus performed the first in vivo human intervention study (an in vivo study is one that utilizes an intact organism, rather than cells or chemicals dissociated from the organism) measuring the interaction between vitamins A and D, in which they found vitamin D to raise serum calcium and vitamin A to lower serum calcium. Since neither vitamin affected the rate of bone resorption or the amount of calcium in the urine, they concluded that the changes in serum calcium resulted from changes in the rate of intestinal calcium absorption. Since intake of vitamins A and D simultaneously did not lower the amount of metabolites of each vitamin in the blood compared to taking one or the other alone, they concluded that vitamin A probably antagonizes the effect of vitamin D by some mechanism other than interfering with the absorption of vitamin D.27 In the rat,33, 34 the decrease in serum calcium is accompanied by an increase in serum phosphorus, but Johansson and Melhus did not measure serum phosphorus levels in humans.
Does Vitamin A “Interfere With” Vitamin D?
Researchers have made several suggestions about possible mechanisms by which vitamin A might interfere with the function of vitamin D. For the ambitious reader, a discussion of the molecular details of these mechanisms is presented in Appendix 2.
Although the net effect of vitamin A is to promote bone resorption and the net effect of vitamin D is to inhibit bone resorption, each vitamin also plays a role in the opposing process: mice that have no vitamin D receptor lose their ability to engage in bone resorption,28 and one study showed vitamin A to inhibit bone resorption.6 Vitamin A also increases the body’s production of growth factors, some of which stimulate osteoblasts and thus bone growth.12 Therefore, it is overly simplistic to say that the two vitamins are “antagonistic” in this respect.
Likewise, although it is true that when vitamins A and D are administered together, D tends to lower serum phosphorus and raise serum calcium, while A tends to lower serum calcium and raise serum phosphorus, the molecular mechanisms of this process are not understood. Vitamin D is necessary for the absorption of both minerals from the intestine,29 so it could be that vitamin A acts as a modulator of vitamin D, controlling to what degree it enhances the uptake of one mineral versus the other.
Thus, the apparent “antagonistic” actions of vitamins A and D are not clear examples of certain antagonism, and the relevant mechanisms by which vitamin A could be said to actively interfere with the function of vitamin D are, as shown in Appendix 2, either undemonstrated or disproved.
How Much Vitamin A is Too Much? The Wrong Question to Ask
Now that we’ve established that vitamin A’s role in bone metabolism is a positive one and that its interaction with vitamin D is of a complex character that includes synergism rather than simply antagonism, let’s turn to the hard evidence: human and animal experiments suggest that sufficient vitamin D – something possessed by none of the groups studied in the epidemiological reports that tie vitamin A to osteoporosis – nullifies the effect of vitamin A in promoting poor skeletal health. Put another way, it isn’t vitamin A in and of itself that contributes to poor skeletal health; it is the combination of comparatively high vitamin A and deficient vitamin D.
Therefore, the question being asked by these epidemiological studies – namely, “how much vitamin A is too much?” – is entirely the wrong one.
There are three basic models we could use to assess the effect of a given amount of vitamin A. The first is to consider the absolute quantity. The second would be a ratio model, wherein the importance of the absolute quantity of vitamin A is subject to the importance of the ratio between vitamins A and D. The third is a threshold or “switch” model, wherein the association between vitamin A and osteoporosis could be “turned on” by deficient vitamin D levels, and likewise “turned off” by vitamin D levels meeting a certain level of sufficiency, just like a light switch. Several groups of researchers have published studies investigating the effects of varying combinations of vitamins A and D on the absorption of calcium and phosphorus, serum levels of these minerals, bone mineral density, other measures of skeletal health, or some combination thereof, in animals and humans, all of which support either a ratio model or a switch model and none of which appear to support a quantity model.
Human Evidence
As noted earlier, Myrhe and others conducted a meta-analysis of all hypervitaminosis A case reports that were published by the year 2000, which established in humans the principle that the toxicity of vitamin A depends not only on the intake of vitamin A, but also on the intake of vitamin D. Although the analysis clearly established this principle, it did not shed any light on whether this interaction constitutes a ratio or a switch model. The researchers found that the median dose of vitamin A reported to be toxic was 175,000 IU higher if vitamin D was also being supplemented, but they did not analyze the actual amount of vitamin D taken, thus making it impossible to ascertain which of the above models the data best supports. Moreover, although 46 percent of the toxicity cases involved elevated blood calcium levels, likely due to an excess of vitamin A-stimulated bone resorption (and thus an efflux of calcium from bone to the blood), the effect of vitamin D supplementation on neither this nor any other criterion of toxicity specific to skeletal health was studied.15
In 2001, Sara Johansson, who hypothesized in her PhD thesis that excess vitamin A contributes to osteoporosis by aggravating the effects vitamin D deficiency,6 and her tutor, Hakan Melhus, who published the first study associating vitamin A intake with the risk of hip fracture,7 together published a double-blind crossover study demonstrating that vitamins A and D have antagonistic effects on serum calcium levels in humans. This is, so far, the only controlled human intervention study to my knowledge that has investigated the interactive effects of vitamins A and D on the skeletal system. Johansson and Melhus found that 50,000 IU of vitamin A as retinyl palmitate decreased serum calcium levels, while 2 µg (micrograms, or millionths of a gram) of calcitriol, or activated vitamin D, increased serum calcium levels.
(By weight, 2 µg of calcitriol is equivalent to 6.66 IU of cholecalciferol, or non-activated vitamin D, but since IU is a measure of physiological effect and the physiological effect per unit of weight of calcitriol is much more powerful than that of cholecalciferol, we can’t express the amount calcitriol in terms of IU.)
Serum calcium levels rose when vitamins A and D were administered together, but less than they did when vitamin D was administered alone. Although an increase in the rate of bone resorption would have increased serum calcium, neither vitamin changed the rate of bone resorption. Likewise, although one vitamin could have exerted an antagonistic effect on the other by blocking its absorption, neither vitamin appeared to affect the absorption of the other. Finally, although a decrease in the rate of excretion of calcium from the blood into the urine would raise serum calcium, neither vitamin changed the amount of calcium in the urine. Johansson and Melhus concluded, therefore, that the difference in serum calcium concentrations produced by different combinations of vitamins A and D is probably due to changes that they produce in the intestinal absorption of calcium.27
Figure 2

Reproduced from J Bone Miner Res 2002;17:1349-1358 with permission of the American Society for Bone and Mineral Research The lines with X’s represent the effect of vitamin D administered alone; the lines with diamonds represent the effect vitamin A administered alone; the lines with circles represent the effect of the placebo control; the lines with triangles represent the effect of vitamins D and A administered together. Asterisks indicate that the effect is statistically significantly different from the effect of the placebo; a cross indicates that the effect is statistically significantly different from the effect of vitamin D alone. A. Vitamin A alone depresses serum calcium relative to the placebo control, while vitamins A and D administered together raise serum calcium almost as much as vitamin D does alone. B. Vitamin D lowers levels of parathyroid hormone (PTH), a sign of vitamin D deficiency, relative to the control. Vitamin A alone does not affect PTH levels, but vitamins A and D administered together lower PTH levels equally as well as does vitamin D alone.
Using the graph published by Johansson and Melhus reproduced in Figure 2, I estimate that serum calcium fell 0.77 percent compared to the placebo group when vitamin A was administered alone, rose 3.25 percent when activated vitamin D was administered alone, and rose 2.87 percent when vitamins A and D were administered together.
Since this study only tested one quantity of each vitamin, it is impossible to know whether it fits a ratio model or a switch model, but one thing is clear: the absolute quantity of vitamin A is irrelevant. In fact, the effect of vitamin A on serum calcium levels was so dependent on the vitamin D status of the individuals that it gave the appearance of having the opposite effect when administered alone, of lowering serum calcium, than it did when administered together with vitamin D, of raising serum calcium.
While comparing the effect of vitamins A and D together to the effects of vitamins A and D alone helps us distinguish the effects of each – something that’s interesting in an academic sense – those of us eating a diet rich in all vitamins across the board are interested in the more practical knowledge of whether the vitamin A component of a nutrient-dense diet is harmful. Clearly, as can be seen by the graph in Figure 2, the lesson of the Johansson and Melhus study is that vitamins A and D together are very effective at raising serum calcium levels. A look through the more extensive animal evidence will reassure us further of the safety and benefit of a nutrient-dense diet.
Animal Evidence: Rats
One group of researchers led by Cynthia Rhode investigated the interaction of dietary vitamins A and D2 in rats.33 Vitamin D2 is a type of vitamin D that is synthesized from ergosterol, a chemical found in plant fats, and is not normally found in significant quantities in the diet. It is worthless in birds because it has no affinity for the protein that stores and carries vitamin D in the blood; it effectively treats rickets and severe vitamin D deficiency in mammals, including humans, but it is up to 10 times less effective than vitamin D3 at maintaining long-term vitamin D status in humans, which is necessary for a variety of other parameters of health.34a
The researchers fed 21-day-old rats a diet deficient in phosphorus, designed to produce rickets. They provided five doses of vitamin A ranging from 0 to about 29,000 IU per day, which is the bodyweight-adjusted equivalent of a 75-kg human taking about 39,000,000 IU of vitamin A per day. They provided six doses of vitamin D2 ranging from 0 to about 26 IU per day, which is the bodyweight-adjusted equivalent of a 75-kg human taking about 35,000 IU of vitamin D per day. When no vitamin D2 was administered, the lowest dose of vitamin A, equivalent to about 52,000 IU for a 75-kg human, increased bone mineralization, while higher doses of vitamin A decreased bone mineralization. For all other doses of vitamin D2, raising the dose of vitamin A progressively lowered the total amount of bone mineralization. Vitamin A also progressively lowered the bone mineral density (which is the amount of mineralization for a given volume of bone, rather than the total amount of mineralization), except at the level approaching the human equivalent of 40 million IU per day, which caused a marked reduction in growth, allowing bone mineral density to stay constant as less minerals were deposited into a smaller femur.
This study clearly refutes a model wherein the absolute quantity of vitamin A is the only operative factor. Whereas when vitamin D2 was held constant, increasing vitamin A decreased bone mineralization, I have assembled data in Table 1 showing that when vitamin D2 was allowed to increase with vitamin A, increasing vitamin A instead increased bone mineralization. Since this study showed vitamin A to have an antagonistic effect even at the highest level of vitamin D2, however, it supports a ratio, rather than a switch, model.
Table 1
Only a portion of the data is shown, simply to elucidate the relative unimportance of absolute quantities of vitamin A when analyzed alone. Vitamin intakes are expressed as bodyweight-adjusted equivalents for a 75-kg human.
| Vitamin D2 (IU/75 kg/day) | Vitamin A (IU/75 kg/day) | Total Femur Ash (mg) |
| 0 | 0 | 30.5 |
| 0 | 51,818 | 34.5 |
| 284 | 15,657,273 | 36.7 |
| 1418 | 39,145,909 | 46.5 |
By contrast, the same group of researchers published a more elaborate and much more useful study last year that supports a switch, rather than a ratio, model. The researchers tested how different combinations of vitamins A and D interact to affect serum calcium and phosphorus concentrations. They used two forms of vitamin A, retinyl acetate and the activated hormone all-trans retinoic acid (ATRA), and four forms of vitamin D: vitamin D2, vitamin D3, the activated hormone calcitriol, and a synthetic variant of calcitriol.34 Unlike in the first study, the researchers used a diet with normal calcium and phosphorus concentrations in this study, making it more relevant to the practical question of how much vitamin A to include in a healthy diet, rather than the academic question of how the two vitamins interact within a disease-producing diet.
When rats consumed an amount of vitamin D2 equivalent to a daily human dose of just under 500 IU, vitamin A as retinyl acetate decreased serum calcium and increased serum phosphorus; yet at an amount of vitamin D2 equivalent to a daily human dose of just over 900 IU, even an amount of vitamin A exceeding the daily human equivalent of 5,000,000 IU could not lower serum calcium levels, although vitamin A continued to raise serum phosphorus levels. In fact, when the amount of vitamin D2 was equivalent to a human dose of about 1400 IU per day, the amount of vitamin A equivalent to a human dose of over 5,000,000 IU per day substantially raised both serum calcium and serum phosphorus levels (Table 3).
Likewise, activated retinoic acid was only able to decrease serum calcium and increase serum phosphorus at low doses of activated calcitriol. When rats consumed higher doses of calcitriol, this ability of retinoic acid was “turned off” like a light switch.
Unfortunately, the researchers only tested one dose of vitamin D3. When rats were fed an amount of vitamin D3 equivalent to a daily human dose of about 500 IU, amounts of vitamin A equivalent to daily human doses of 2,600,000 IU and 5,000,000 IU both lowered serum calcium and raised serum phosphorus. An amount of vitamin A equivalent to a daily human dose of just over 17,000 IU, however, caused a small (but not statistically significant) rise in serum calcium, and actually caused a statistically significant decrease in serum phosphorus levels.
Since vitamin D3 (shown in Table 2) was only administered at one dose that was insufficient to “flip the switch,” the data first appear to support a ratio model. The vitamin D2 data presented in Table 3, however, establish an unambiguous switch model.
Table 2
When vitamin D3 was supplied at an amount below the switch threshold of 938 IU, it exhibited a ratio model, but if one compares the two values for 17,255 IU of vitamin A, it becomes clear that the absolute amount of vitamin A is irrelevant. Vitamin intakes are presented as bodyweight-adjusted equivalents for a 75-kg human.
| Vitamin A (IU/75 kg/day) | Vitamin D3 (IU/75 kg/day) | Serum Calcium (mM) | Serum Phosphorus (mM) |
| 17,255 | 0 | 1.10 | 3.35 |
| 0 | 454 | 2.15 | 3.06 |
| 17,255 | 454 | 2.30 | 2.41 |
| 2,603,455 | 454 | 2.08 | 2.84 |
| 5,146,364 | 454 | 1.73 | 3.23 |
Table 3
When vitamin D2 was supplied below the switch threshold of 938 IU, vitamin A decreased serum calcium. The very small decrease in the average serum calcium that occurred when 938 IU of vitamin D2 was administered was not statistically significant. Once the “switch” was “flipped,” vitamin A actually enhanced the rise in serum calcium.
| Vitamin A (IU/75 kg/day) | Vitamin D2 (IU/75 kg/day) | Serum Calcium (mM) | Serum Phosphorus (mM) |
| 17,255 | 0 | 1.23 | 3.74 |
| 0 | 469 | 1.95 | 2.65 |
| 5,206,909 | 469 | 1.40 | 4.58 |
| 0 | 938 | 2.33 | 2.87 |
| 5,206,909 | 938 | 2.25 | 3.87 |
| 0 | 1407 | 2.20 | 2.81 |
| 5,206,909 | 1407 | 2.50 | 3.45 |
Animal Evidence: Broiler Chickens
A team of researchers led by Whitehead published a report in 2004 showing that at various feed concentrations of vitamin D3, changing the feed concentration of vitamin A from 2.4 mg/kg to 4.5 mg/kg had no effect on bone mineralization, bone strength, or serum calcium in broiler chickens.35 The authors did not report how much food was consumed by each chicken per day, but I used data from another study,36 to estimate that the two doses of vitamin A were equivalent to bodyweight-adjusted daily human doses of about 69,000 IU and 130,000 IU. Although this study found no antagonistic effect of vitamin A at any level of vitamin D, the minimum vitamin D intake in the study was equivalent to a daily human dose of over 1700 IU. This minimum vitamin D intake is well above the vitamin D intake (938 IU per day) that turned off the antagonistic effect of vitamin A for rats in the previous study. Thus, this study is consistent with a switch model, wherein the researchers administered sufficient vitamin D to turn off the negative effect of vitamin A to all treatment groups.
Aburto and Britton published two earlier studies in 1998 using the same breed of broiler chicken, both of which support a switch model, yet also establish the threshold of vitamin D3 necessary to flip the switch as considerably higher than that established by the Rhode and Whitehead studies. In the first,37 increasing the amount of vitamin A from an estimated38 equivalent of a daily human dose of about 12,000 IU to almost 370,000 IU statistically significantly reduced bone ash, but only when the chickens were shielded from all UV light and fed the equivalent of a daily human dose of about 4000 IU of vitamin D3. At the equivalent of a daily human dose of about 21,000 IU of vitamin D3, on the other hand, vitamin A did not have a statistically significant effect. When the chicks were exposed to UV light, the dose of vitamin A had no effect on bone ash and no interaction with vitamin D at any dose of either vitamin.
Since there was no statistically significant difference in bone ash among any of the six different vitamin A-to-D ratios consumed by chickens exposed to UV light, this study supports a switch model, wherein UV light, as well as some dietary amount of vitamin D3 between the human equivalents of 4000 and 21,000 IU per day, provides a sufficient level of vitamin D to turn off the effect of vitamin A. Although the Whitehead study found a much lower vitamin D dose was sufficient to “turn off” the antagonistic effect of vitamin A in the same breed of chicken, the authors of that study provided twenty-three hours of light per day. They didn’t specify what type of lighting they used, but since they took no deliberate measure to shield their chicks from UV light, the lighting may have provided the chicks may with additional vitamin D.
In the second study by Aburto and Britton,39 the interaction between vitamins A and D was studied using three types of vitamin D: vitamin D3, the semi-activated metabolite, 25 (OH) D, and the fully activated metabolite, calcitriol. The researchers shielded the chicks in all groups from UV light. For any given amount of vitamin D3, raising the amount of vitamin A from an estimated40 equivalent of a daily human dose of about 12,000 IU to that that of about 370,000 IU decreased bone mineralization and increased the incidence of rickets. This was true even at an amount of vitamin D3 equivalent to a daily human dose of over 26,000 IU.
At first impression, this study seems to support a ratio model. Yet the doses of vitamin A used in the high-vitamin A and low-vitamin A groups were so disparate that it is impossible to compare equivalent vitamin A-to-vitamin D ratios between the two groups: the highest vitamin A-to-vitamin D ratio in the low-vitamin A group was 7.5, while the lowest vitamin A-to-vitamin D ratio in the high-vitamin A group was 14. For reference, high-vitamin cod liver oil has a vitamin A-to-vitamin D ratio of 10. Even though the aforementioned high-vitamin A dose had almost twice the A-to-D ratio of the aforementioned low-vitamin A dose, it actually yielded a 24 percent increase in bone mineralization and a 77 percent decrease in the incidence of rickets. This shows quite clearly that, as long as the ratios are within a reasonable range, it is better to have more of both vitamins – as one would get from high-vitamin cod liver oil – than to have less of both vitamins.
Once we consider the interaction of vitamin A with the semi-activated and activated metabolites of vitamin D, it becomes clear that this study conforms to a switch model. The semi-activated metabolite, 25 (OH) D, which is about twice as active as vitamin D3 in broiler chickens, at an amount equivalent to a daily human dose of almost 3,000 IU,40a was able to completely turn off the association between vitamin A and the severity of rickets, while an amount equivalent to a daily human dose of almost 6,000 IU was able to completely turn off the association of vitamin A with increased incidence of rickets and decreased bone ash. Likewise, the fully activated metabolite, calcitriol, which is about ten times as active as vitamin D3 in broiler chickens, at an amount equivalent to a daily human dose of about 2,600 IU, was able to completely turn off the association between vitamin A and the incidence and severity of rickets. Vitamin A did not affect bone mineralization when fed in conjunction with any dose of calcitriol.
The three studies with broiler chickens taken together support a switch model, where sufficient vitamin D turns off the association between vitamin A and bone defects. The most compelling evidence for this is that UV light, which if provided in sufficient quantity is able to fully provide the exact need for vitamin D,17 was able to completely abolish the negative effect of vitamin A.37 Yet the dietary dose required to turn off the switch in the absence of UV light – equivalent to a daily human dose of 20,000 IU – was surprisingly high. This is probably because, as explained by the authors of the Whitehead study, modern breeds of broiler chickens have a genetically increased need for calcium. The researchers only provided the chicks with 75 percent of the calcium that modern broilers need. Suboptimal amounts or ratios of calcium and phosphorus can increase the need for vitamin D in broiler chickens by up to eight times – one reason that rickets and other signs of calcium and vitamin D deficiencies frequently manifest themselves among broiler chickens in commercial conditions, even though the diets of these chickens are often fortified with large amounts of these nutrients.35
Rhode and Deluca showed that a mere amount of vitamin D equivalent to a daily human dose of just over 900 IU was able to turn off the negative effect of vitamin A on the serum calcium levels of rats, who as mammals are closer to humans than are chickens, even when the rats consumed amounts of vitamin A that exceeded the equivalent of a daily human dose of 5,000,000 IU.34
This amount of vitamin D needed is equivalent to less than a quarter of the human dose recommended by the Vitamin D Council, which suggests that following the Council’s vitamin D recommendations may in itself turn off any negative effect of vitamin A in humans. That same dose equivalent is, on the other hand, twice the amount of vitamin D that participants in the Nurses’ Health Study were consuming in the highest quintile and six times the amount they were consuming in the lowest quintile, while it is five times the amount of vitamin D that residents of Uppsala, Sweden were consuming in the highest quintile and 10 times what they were consuming in the lowest quintile. This suggests that the studies associating vitamin A with osteoporosis in these populations may bear no relevance to those of us consuming even barely sufficient amounts of vitamin D. Since vitamin A actually enhanced the rise in serum calcium levels when the rats consumed a somewhat higher amount of vitamin D, it could be the case that vitamin A may help prevent osteoporosis in populations that consume sufficient vitamin D.
Towards a New Understanding of Vitamin Toxicity: Balance versus Amount
By now it should be clear that a high level of vitamin A does not, in and of itself, cause osteoporosis. Vitamin A can only exert the harmful effects attributed to it against the backdrop of vitamin D deficiency, meaning that is not an excess of vitamin A that contributes to osteoporosis, but an imbalance between vitamins A and D.
Although vitamin A exerts the effects believed to contribute to osteoporosis at levels that are well below what is conventionally understood as “toxic,” the very principle that its effects are modified by vitamin D calls into question the conventional understanding of vitamin toxicity itself. If certain vitamins must be taken in a certain balance, are the toxic effects of one given in excess, out of proportion to the others, attributable to an excess of that vitamin, or instead attributable to a relative deficiency of the other vitamins that must accompany it?
I am by no means the first writer to suggest such an interaction. Researchers made the first intimation of such an interaction in 1936, when Tabor showed high doses of carotenes to interfere with the availability of vitamin D to treat rickets in cows.34 Numerous animal studies have shown that vitamin A reduces the toxicity of vitamin D and vitamin D reduces the toxicity of vitamin A.6 Yet, in 2003 Myhre and others found only 81 cases of hypervitaminosis A out of 259 that even reported whether or not vitamin D was being supplemented,15 few of the epidemiological studies on vitamin A and fracture risk report vitamin D intakes, and rare is it for health experts warning of the harms of “excess” vitamin A to note that its safety depends on the concomitant intake of vitamin D.
A final animal study41 I will discuss, published in 1985 by a team of researchers led by Metz, drives home the point that the toxicity of each of vitamins A and D depends on the amount supplied of the other as much as the amount supplied of itself. The researchers fed turkey poults diets that were either deficient in both vitamins, sufficient in both vitamins, excessive in vitamin A, excessive in vitamin D, or excessive in both. The researchers fortified the excess vitamin A diet with 400,000 IU of vitamin A per kg of food and the excess vitamin D diet with 900,000 IU of vitamin D3 per kg of food. The authors didn’t report how much food the turkey poults ate, but they estimated that the high vitamin A group consumed 15,000 IU per day, which is, after adjusting for body weight, equivalent to a human taking in over 3,500,000 IU per day. Since the turkey poults in the other groups weighed almost twice as much, it is possible that they consumed twice as much food, which means the group fed high amounts of both vitamins consumed equivalent human doses of between 3.,500,000 and 7,000,000 IU per day of vitamin A, and between 8,000,000 and 16,000,000 IU per day of vitamin D. The diets were fed for 25 days.
In the groups fed either the deficient diet or the diet high in vitamin A, the birds had retarded growth, lameness, and were eventually unable to walk. In the high-vitamin A group, the birds had lower bone mineral density, thin bones, various skeletal lesions, poorly defined growth zones within the bones, and spontaneous fractures. In the high-vitamin D group, the birds had swollen, pale kidneys and mineral depositions within the kidneys. Yet in the group fed high amounts of both vitamins, all of the toxic effects of both vitamins completely disappeared!
We shouldn’t conclude that it is necessarily safe for humans to take the same amounts of vitamins fed to the turkeys in this study, because turkeys and humans do not necessarily have the same needs for vitamins, and the duration of the study was only 25 days. Nevertheless, the study clearly demonstrates that the old paradigm in which the toxicity of a vitamin is a function of that vitamin alone is false, and that a new paradigm – that the toxicity of a vitamin depends on its balance with other vitamins – which has been advanced in the background by various researchers over the last seventy years, is necessary.
Interactions Between Vitamins A, D, E, and K
Research has not only demonstrated an interaction between vitamins A and D, but has also demonstrated interactions between all of the fat-soluble vitamins. When researchers feed rats vitamin A at 10 times the normal amount, serum levels of vitamins E and D drop; when they feed rats vitamin A at 50 times the normal amount, serum levels of vitamin K also drop. One symptom of both vitamin A and vitamin E toxicity in the rat – internal hemorrhaging – resembles a major symptom of vitamin K deficiency, suggesting that part of the toxicity of vitamins A and E may be due to an imbalance with vitamin K. One study has shown interactions between vitamins A, D, E, and K in chickens.42 Like vitamin A, Aburto and Britton also showed extreme doses of vitamin E to reduce bone ash and plasma calcium and to increase rickets in chickens – effects all remedied by feeding high doses of vitamin D.37
Several studies, when taken together, suggest that vitamin E can interfere with the functioning of, or increase the need for, vitamin A. In Nutrition and Physical Degeneration, Weston Price described the importance of vitamin A for the development of eye tissue and cited experiments carried out by Fred Hale, wherein vitamin A-deficient pigs gave birth to offspring with a variety of eye defects, including blindness and in some cases the absence of eyeballs.43 In a more recent study,44 researchers injected the environmental pollutant TCDD, the most potent dioxin, vitamin E as a-tocopherol succinate, and vitamin A as retinol acetate, into eggs containing chicken embryos, and then let the chicks hatch. Vitamin A and vitamin E both protected against TCDD-induced birth defects, but vitamin E caused birth defects of its own – mostly eye abnormalities. One chick, like some of Fred Hale’s vitamin A-deficient pigs, was even born with no eyes.
TCDD, which acts as a hormone and both mimics vitamin A and opposes vitamin A in various ways,45 helped reduce the vitamin E-induced birth defects, but the researchers did not test whether or not vitamin A protected against these birth defects. In humans, vitamin A helps improve the eye disease retinitis pigmentosis, while vitamin E accelerates it.46 If high doses of vitamin E can induce the same eye defects as induced by deficient doses of vitamin A, and if vitamin E accelerates the same eye diseases as are helped by vitamin A, then vitamin E’s toxic effects on the eye may result from a depletion of vitamin A. In fact, excessive doses of the a-tocopherol form of vitamin E not only interfere with the functioning of vitamin A, but actually interfere with the functioning of vitamin E itself, by inhibiting the action of the other important parts of the vitamin E complex.47
These findings all demonstrate the importance of a nutrient-dense diet as a whole, wherein nutrients naturally occur in balanced proportions, and the folly of laying blame for toxicity on a vitamin in and of itself without taking into account its interaction with other vitamins.
From Vitamins Back to Foods
The fatal flaw of the theory that vitamin A causes osteoporis is paradigmatic: the general approach to nutrition that looks at vitamins as isolated chemicals acting in a vacuum, rather than foods – grand, complex associations of many chemicals that all act in concert – can only bear so much fruit before faltering. Reductionism does indeed have its place. There is true value in performing experiments to see which effects we can attribute to vitamin A and which we can attribute to vitamin D. The utter folly, however, of the conclusion that because vitamin A “antagonizes” vitamin D, we should avoid it, is revealed in the history of the discovery of vitamins.
The first cure for rickets was not vitamin D. The first cure for rickets was a food: cod liver oil. Cod liver oil was initially used as a therapeutic agent in the 1770s, and by the mid-nineteenth century, it was well-recognized as a cure for rickets, osteomalacia, general malnourishment, and various eye conditions.61 Vitamin A was first discovered in 1913 as a component of cod liver oil and butter fat. Even though vitamin A is present in cod liver oil at concentrations 10 to 25 times those of its supposed enemy, vitamin D, cod liver oil was such an effective cure for rickets that the British physician Sir Edward Mellanby attributed the antirachitic properties of cod liver oil to its vitamin A.33
In 1921, however, Shipley, Park, McCollum and Simmonds published a landmark paper demonstrating that butterfat, fed as five percent of the diet, was not sufficient to treat rickets, unlike smaller doses of cod liver oil.62 (It should be kept in mind that even grass-fed butter is very low in vitamin D during a “vitamin D winter,” or when cows are fed grass indoors.) This was the first paper suggesting that the factor that treated xerophthalmia – an eye condition caused by vitamin A deficiency – was a separate factor from that which treated rickets, due to their different distribution among foods. In 1922, the same researchers showed that cod liver oil aerated at the temperature of boiling water for 12 to 20 hours retained its antirachitic property, but lost its effectiveness against xerophthalmia, confirming the separate identity of these two factors, the second of which came to be known as vitamin D. Yet cod liver oil was effective against diseases of both vitamin A deficiency and vitamin D deficiency. The two vitamins did not, and do not, cancel each other out.
We can see a similar phenomenon in the way Weston Price demonstrated the high antirachitic activity of his high-vitamin butter oil. He fed two rats a diet deficient in calcium and phosphorus, and fortified one rat’s diet with the butter oil. The latter rat not only recovered from rickets, but experienced a simultaneous rise in both serum calcium and phosphorus – an effect of multiple compounds working in concert within the vehicle of a single food.63
Eating whole foods, however, is not enough. The so-called “primitives” studied by Weston Price had adapted complete diets and lifestyles to suit their needs. Foods rich in one nutrient and poor in another were combined together into a diet that, as a whole, yielded the high amounts of minerals and vitamins that Price documented. Thus, the finding that subjects in the Nurses’ Health Study who regularly ate liver – a traditional whole food – had an increased risk for fracture is actually consistent with Price’s findings. By itself, liver from land animals, especially ruminants like cattle and lamb, is very rich in vitamin A,64 while livers from both ruminants and poultry animals have only about 12 IU of vitamin D for every 100 grams.65
Cod liver oil, on the other hand, is rich in both vitamins A and D. In fact, high-vitamin cod liver oil, as used by Price, not only contains more than twice the amount of vitamin A, but also contains almost six times the amount of vitamin D as regular cod liver oil, lowering the vitamin A-to-D ratio from 25 to 1 to 10 to 1, and can provide 4,000 IU of vitamin D itself in just over a tablespoon.66,67 In Norway, where the vitamin D winter is long, many traditional coast-dwelling villages still consume a dish called molje, which is made from both fish liver and fish liver oil, either cod or saithe, depending on the season. Although fish livers are very rich in vitamin A, a typical meal of molje will provide just less than 3000 IU of vitamin D, while people with larger appetites may consume nearly 6000 IU of vitamin D in a single meal.68
It makes sense, then, that high-vitamin cod liver oil should serve as a safe standalone supply of vitamin A even during the winter, while liver from land animals should either be used during seasons where the supply of UV-B rays from the sun is rich enough to guarantee adequate vitamin D, or in conjunction with vitamin D-rich foods such as lard from pastured pigs, oily fish, shellfish, and egg yolks from pastured chickens, all of which supply smaller but substantial amounts of vitamin D. (For a list of these and other food sources of vitamin D with specific concentrations listed, see Appendix 3.
Major nutrients like vitamins A and D, calcium, and phosphorus, are not the end of the osteoporosis horizon. Studies suggest a role for minor nutrients and trace metals, including zinc, vitamin B12, folate, boron, silicon, manganese, zinc, and copper.69 In Soil, Grass, and Cancer, Andre Voisin wrote of cattle that grazed in the Everglades on copper-deficient soil. Despite sufficient calcium and phosphorus in the diet, these cattle developed rickets and osteomalacia, which was remedied by providing dietary copper.70 Likewise, Robert Becker described his own experiments in The Body Electric that suggested that copper is the glue holding the hydroxyapatite crystals – calcium and phosphorus salts – to the collagen matrix of bone.71
The healthy populations studied by Weston Price not only ate combinations of foods that yielded ideal nutrition but also engaged in specific practices to maintain the fertility of their soil, which would guarantee the provision of such trace metals in addition to the major nutrients. In fact, Price considered soil fertility such an important factor in the health of the indigenous populations whom he studied that he invited the prolific soil scientist, William Albrecht, to write the second chapter to the supplement of Nutrition and Physical Degeneration entitled “Food is Fabricated Soil Fertility” – the only chapter of this epic book that was written by someone other than Price.
It is just as important, then, to seek out foods produced by farming methods designed to produce nutritious crops, rather than high yields, as it is to seek out the right types of foods. Additionally, as soils are lost to runoff eventually draining into the sea, it becomes more and more important to emphasize sea foods in our diet, such as unrefined sea salt, fish, shellfish, and sea vegetables, which are grown in the ever-richer “soils” of the ocean.
It is, then, a holistic paradigm that takes pause and backs away from the minutia for a moment to see the grand picture – in which vitamin A is but one part of a nutrient-dense diet – that is able to truly advance our understanding of how to achieve health. When vitamin A is divorced from this complete approach to health, added to milk products in lands long-eclipsed by vitamin D winters, consumed as liver in the absence of other foods that a traditional diet would provide for balance, or fed to animals at doses vastly out of proportion to the other nutrients that the animals would encounter in their natural diets, it does indeed contribute to a degeneration of bone health. Yet, as Price documented, the most superb examples of vibrant skeletal health are populations that ate diets very rich in preformed vitamin A. When vitamin A is part of a nutrient-dense, traditional diet, it does not cause osteoporosis, but instead confers upon those who consume it vibrant health, energy, and resistance to degenerative diseases.
Appendix 1: Vitamins A and D Help Regulate Bone Remodeling
The activated form of vitamin A, retinoic acid (RA), and the activated form of vitamin D, calcitriol, are both hormones. A hormone is a chemical that carries out various cell signaling functions by first binding to a receptor, and thus causing a change in the shape of that receptor, which in turn causes the receptor to either initiate a cascade of enzymes and other signaling molecules that carry out some direct effect in the cell, or to move into the nucleus where it, usually complexed with one or more other molecules, binds to specific parts of genes and causes them to alter their expression – to turn them on, turn them off, turn them up, or turn them down. The effect of any given hormone may vary widely between different cells and tissues, because it is determined as much by the specific receptor to which the hormone binds and the presence or absence of many different subsequent signaling molecules that may be activated as a result of that binding as it does on the hormone itself.
Activated vitamin A must bind to one of at least three receptors called the retinoic acid receptors (RARs) to carry out its function. After binding to an RAR, these two molecules together must bind to a retinoid X receptor (RXR), after which this triple-complex binds to specific parts of specific genes called “retinoid acid response elements” (RAREs), and then proceeds to alter the expression of particular genes, which are referred to as retinoic acid’s “target genes.”6
Similarly, activated vitamin D must bind to the vitamin D receptor (VDR) and subsequently complex with an RXR in order to bind to vitamin D response elements (DREs) and alter the expression of vitamin D target genes.6
Both osteoblasts72,73 and osteoclasts6 contain both RARs and the VDR; thus vitamins A and D both regulate the activity of osteoblasts and osteoclasts. In some cases, their regulatory effect involves interactions between the vitamins and their respective receptors on the actual bone cells. In other ways, the vitamins modify bone remodeling indirectly by affecting mineral homeostasis.28
The process of bone remodeling involves a synergism between osteoblasts and osteoclasts, wherein the two regulate each other. The bone remodeling process is initiated when immature osteoblasts secrete receptor activator of nuclear factor-kB ligand (RANKL), which binds to receptor activator of nuclear factor-kB (RANK), a receptor on the surface of osteoclast precursor cells, thus activating them to mature into osteoclasts and begin eroding bone. As osteoclasts erode bone, the eroded bone releases growth factor signals such as insulin-like growth factor-1 (IGF-1) and transforming growth factor-ß (TGF-ß) that stimulate the maturation of osteoblasts. As osteoblasts mature, they progressively secrete less RANKL and begin secreting osteoprotegrin (OPG), which binds to the RANK receptor and prevents RANKL from activating it. As the balance of signals secreted by the maturing osteoblasts increasingly favors OPG over RANKL, osteoclast activity increasingly slows until it finally stops and gives way to the process of bone growth, conducted by the fully matured osteoblasts.6
Activated vitamin A increases bone resorption by increasing the expression of RANKL and decreasing the expression of OPG. The rate of bone resorption is determined by the ratio of RANKL, which activates RANK, to OPG, which competitively inhibits RANKL from activating RANK.6
Studies generally show that the dominant effect of vitamin A is to promote bone resorption, while the dominant effect of vitamin D is to inhibit bone resorption. One study showed, however, that vitamin A can inhibit bone resorption;6 likewise, vitamin D is required for bone resorption, which it promotes indirectly by increasing calcium and phosphorus absorption in the intestines.28 Vitamin A can also contribute to bone growth by raising the level of growth factors, which activate osteoblasts.12
Although it may sound confusing that vitamin A and vitamin D exert opposing actions, other chemicals are also known to have conflicting roles in regulating bone remodeling. For example, leptin – now becoming popularly known as an appetite regulator – was first discovered to be an inducer of bone resorption produced by nerve cells, but may also be secreted by bone tissue itself as an inducer of bone growth.6 Since bone resorption and growth are in some ways antagonistic processes, yet nevertheless complimentary processes that form part of the same system and synergistically regulate each other, it is unsurprising that the various external factors that affect this regulation may have conflicting roles.
The folly of the claim that one or another of the vitamins is bad because it antagonizes the other is revealed by the fact that, in the ways described above, vitamin A actually “antagonizes” vitamin A itself, and vitamin D actually “antagonizes” vitamin D itself!
Appendix 2: Does Vitamin A “Interfere” With Vitamin D?
Several suggestions have been made about possible mechanisms by which vitamin A might interfere with the function of vitamin D:6
- After each activated vitamin binds to its respective receptor, each must bind to a second receptor called the “retinoid X receptor” (RXR). Since vitamins A and D both must bind to the same secondary receptor, vitamin A could competitively inhibit the binding of vitamin D to the RXR.
- The active form of vitamin A, retinoic acid, can induce the expression of the enzyme 24-OH-hydroxylase, which causes the inactivation of vitamin D.
- Vitamins A and D could compete for absorption in the intestine.
One study showed that the RXR is in short enough supply in a particular type of pituitary cell that vitamins A and D can competitively inhibit each other in this way, but another study showed that the RXR is supplied abundantly in bone marrow cells and that this is not a mechanism of competition for the two vitamins in bone marrow.74 No studies have demonstrated this type of competition in bone or intestinal cells.
Although two studies found that activated vitamin A induced the expression of the 24-OH-hydroxylase enzyme in mice after eight and 18 hours, one study showed that this is simply a temporary flux, and that the subsequent decrease of activated vitamin D actually depresses 24-OH-hydroxylase over the long-term. Vitamins A and D have the same interactive effect on serum calcium and phosphorus levels even when the form of vitamin D used is a synthetic isomer that cannot be destroyed by the 24-OH-hydroxylase enzyme, indicating that this effect has nothing to do with vitamin A encouraging the inactivation of vitamin D.34
One study showed that high levels of vitamin D lowered vitamin A stores in the liver and lowered the level of vitamin A in the blood.39 The researchers suggested that vitamin D was preventing vitamin A from being absorbed and transported in the intestine, but without analyzing fecal loss of vitamin A, they had no way to show that any “interference” was taking place. Another possibility could have been that high vitamin D activity was eliciting the delivery of vitamin A to the tissues in order to be utilized in cooperative processes that require both vitamins. This is supported by a previously published study by the same authors,37 in which exposure of chickens to UV light – which produces vitamin D that does not need to be transported from the intestines to the blood – reduced liver stores and blood levels of vitamin A.
Likewise, Johansson and Melhus found that vitamins A and D had antagonistic effects on serum calcium levels in humans, but that the amount of one vitamin absorbed did not appear to be affected by the administration of the other vitamin.27
On the other hand, there are examples where one vitamin may truly interfere with the other vitamin’s function. For example, stem cells in bone marrow may differentiate into (turn into) either granulocytic cells or monocytic cells, which are two different types of white blood cells. In one particular type of bone marrow cell, retinoic acid induces granulocytic differentiation, while calcitriol induces monocytic differentiation. Calcitriol exerts a dominant negative effect on retinoic acid in this cell, meaning that calcitriol inhibits the action of retinoic acid, but retinoic acid does not inhibit the effect of calcictriol. In this case, part of the mechanism by which calcitriol acts is to actually knock the vitamin A and its receptor right off the gene that it is binding to. In other types of bone marrow cells, however, vitamins A and D both induce the same type of differentiation,74 and in leukemia bone marrow cells, vitamin D adds to the effect of vitamin A in inducing a particular type of differentiation, but has no action by itself.50
Although there are some cases in which one vitamin may interfere with another, this is only true in certain types of cells where the cell has evolved that specific type of regulatory process. In other cases, one vitamin may enhance the other’s action, or even require the other’s presence to carry out its function. We should not second-guess the complex molecular machinery of the cell that is designed to designate functions to each vitamin specific to that cell, functions that vary and cannot be generalized from cell to cell and tissue to tissue.
With respect to osteoporosis, it does not appear that the apparent “antagonism” between vitamins A and D involves any type of molecular “interference” between the two vitamins.
Appendix 3: Food Sources of Vitamin D65
(amounts given are for 100 grams, or about 3.5 ounces.)
| Cod Liver Oil Lard (Pork Fat) Atlantic Herring (Pickled) Eastern Oysters (Steamed) Catfish (Steamed/Poached) Skinless Sardines (Water Packed) Mackerel (Canned/Drained) Smoked Chinook Salmon Sturgeon Roe Shrimp (Canned/Drained) Egg Yolk (Fresh) (One yolk contains about 24 IU) Butter Lamb Liver (Braised) Beef Tallow Pork Liver (Braised) Beef Liver (Fried) Beef Tripe (Raw) Beef Kidney (Simmered) Chicken Livers (Simmered) Small Clams (Steamed/Cooked Moist) Blue Crab (Steamed) Crayfish/Crawdads (Steamed) Northern Lobster (Steamed) |
10,000 2,800 680 642 500 480 450 320 232 172 148 56 |
- Price, Weston A., Nutrition and Physical Degeneration, self-published, (1945) p. 275.
- Nieves, et al., “Primary Osteoporosis,” in Coe and Favus, eds., Disorders of Bone and Mineral Metabolism, Philadelphia: Lippincott Williams and Wilkins, (2002) p. 805.
- Solomons, Noel, “Vitamin A and its role in children’s nutrition and health,” Wise Traditions 2005, November 12, 2005 (lecture).
- Cannel, John, “The Vitamin D Newsletter: Questions and Answers,” http://www.cholecalciferol-council.com/1_06_newsletter.pdf Published January, 2006. Accessed January 22, 2006.
- Council for Responsible Nutrition, “CRN Reaffirms Vitamin A Intake Levels As Safe and Beneficial: Urges Continued Support of IOM Policy on Upper Limits,” http://www.crnusa.org/Shellnr010102.html Published January 1, 2002. Accessed January 2, 2005.
- Johansson, Sara, “Vitamin A and Osteoporosis: Experimental and Clinical Studies,” Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine, 1392 (2004) 65 pp.
- Melhus, et al., “Excessive Dietary Intake of Vitamin A is Associated with Reduced Bone Mineral Density and Increased Risk for Hip Fracture,” Annals of Internal Medicine, Vol. 129 No. 10 (1998) 770-778.
- Ballew, et al., “High Serum Retinyl Esters Are Not Associated with Reduced Bone Mineral Density in the Third National Health and Nutrition Examination Survey, 1988-1994” Journal of Bone and Mineral Research, Vol. 16 No. 12 (2001) p. 2306.
- Feskanich, “Vitamin A and Hip Fractures Among Post-Menopausal Women,” Journal of the American Medical Association, Vol. 287 No. 1 (2002) 47-54.
- Promislow et al., “Retinol Intake and Bone Mineral Density in the Elderly: The Rancho Bernardo Study,” Journal of Bone and Mineral Research, Vol. 17 No. 8 (2002) 1349-58.
- Michaelsson et al., “Serum Retinol Levels and the Risk of Fracture,” New England Journal of Medicine, 348 (2003) 287-94.
- Opotowsky, et al., “Serum Vitamin A Concentration and the Risk of Hip Fracture among Women 50 to 74 Years Old in the United States: A Prospective Analysis of the NHANES I Follow-up Study,” The American Journal of Medicine, 117 (2004) 169-174.
- Lim et al., “Vitamin A intake and the risk of hip fracture in postmenopausal women: the Iowa Women’s Health Study,” Osteoporosis Int, 15 (2004) 552-559.
- Barker et al., “Serum Retinoids and Beta-Carotene as Predictors of Hip and Other Fractures in Elderly Women,” Journal of Bone and Mineral Research, Vol. 20 No. 6 (2005) 913.
- Myhre, et al., “Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations,” Am J Clin Nutr 78 (2003) 1152-9.
- Booth, et al., “Natural food sources of vitamin A and provitamin A,” In: Scrimshaw, ed., Food and Nutrition Bulletin, Vol. 14 No. 1., Tokyo: United Nations University (1992) http://www.unu.edu/unupress/food/8F141e/8F141E04.htm Accessed January 22, 2006.
- Adams and Hollis, “Vitamin D: Synthesis, Metabolism, and Clinical Measurement.” In: Coe and Favus, eds., Disorders of Bone and Mineral Metabolism, Philadelphia: Lippincott Williams and Wilkins (2002) p. 159.
- Webb, et al., “Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin,” J Clin Endocrinol Metab, Vol. 67 No. 2 (1988) 373-8.
- Engelsen, et al., “Symposium-in-Print: UV Radiation, Vitamin D and Human Health: An Unfolding Controversy: Daily Duration of Vitamin D Synthesis in Human Skin with Relation to Latitude Total Ozone, Altitude, Ground Cover, Aerosols and Cloud Thickness,” Photochemistry and Photobiology, 81 (2005) 1287-1290.
- Canty and Associates LLC, “Weatherbase: Historical Weather for Uppsala, Sweden,” http://www.weatherbase.com/weather/weather.php3?s=024580 Accessed January 2,2006
- Engelson, Ola, “Duration of Vitamin D Synthesis in Human Skin: Version 1.0” http://nadir.nilu.no/~olaeng/fastrt/VitD.html (2005) Accessed January 22, 2006.
- Heaney, Robert P., “The Vitamin D requirement in health and disease,” Journal of Steroid Biochemistry & Molecular Biology, 97 (2005)13-19.
- Cannel, John, “Vit D: When Why Where and How Much,” Wise Traditions 2005 November 12, 2005 (lecture).
- Michaelsson, et al., “Dietary calcium and vitamin D intake in relation to osteoporotic fracture risk,” Bone, 32 (2003) 694-703.
- Falahati-Nini, et al., “Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men,” J Clin Invest Vol. 106 No. 12 (2000) 1553-60.
- Mauras et al., “Growth hormone, insulin-like growth factor I and sex hormones: effects on protein and calcium metabolism,” Acta Paediatr Suppl. Vol. 88 No. 433 (1999) 81-3.
- Johansson and Melhus, “Vitamin A Antagonizes Calcium Response to Vitamin D in Man,” Journal of Bone and Mineral Research, Vol. 16 No. 10 (2001) 1899.
- Amling, et al., “Rescue of the Skeletal Phenotype of Vitamin D Receptor-Ablated Mice in the Setting of Normal Mineral Ion Homeostasis: Formal Histomorphometric and Biomechanical Analyses,” Endocrinology, Vol. 140 No. 11 (1999) 4982-4987.
- Favus, Murray J., “Intestinal Absorption of Caclium, Magnesium and Phosphorus.” In: Coe and Favus, eds., Disorders of Bone and Mineral Metabolism: Second Edition, Philadelphia: Lippincott Williams and Wilkins (2002) 48-73.
- Masuyama, et al., “Dietary Calcium and Phosphorus Ratio Regulates Bone Mineralization and Turnover in Vitamin D Receptor Knockout Mice by Affecting Intestinal Calcium and Phosphorus Absorption,” Journal of Bone and Mineral Research, Vol. 18 No. 7 (2003) 1217.
- Nemere, et al., “Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25(OH)2D3-MARRS) and phosphate uptake in intestinal cells,” Proc Natl Acad Sci USA (2004) 7392-7397.
- Rohe, et al., “Indentification and characterization of 1,25D3-membrane-associated rapid response steroid (1,25D3-MARRS)-binding protein in rat IEC-6 cell,” Steroids, 70 (2005) 458-463.
- Rhode, et al., “Vitamin A Antagonizes the Action of Vitamin D in Rats,” J. Nutr., 129 (1999) 2246-2250.
- Rhode and DeLuca, “All-trans Retinoic Acid Antagonizes the Action of Calciferol and Its Active Metabolite, 1,25-Dihydroxycholecalciferol, in Rats,” J Nutr, 135 (2005) 1647-1652.34.a. Armas, et al., “Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans,” The Journal of Clinical Endocrinology and Metabolism, Vol. 89 No. 11 (2004) 5387-5391.
- Whitehead, et al., “High vitamin D3 requirements in broilers for bone quality and prevention of tibial dyschondroplasia and interactions with dietary calcium, available phosphorus, and vitamin A,” British Poultry Science, Vol. 45 No. 3 (2004) 425-436.
- The mean feed intake of all groups of the same breed of broiler chick (Ross x Ross 308) during the same period of time (14 days) and beginning on the same day of age (one day old) in a separate study by other authors was reported as 36.2 grams/day. Knox, Anne, et al., “Confidential Report: Broiler Trial: A Comparison of the Performance of Broiler Chicks Fed on Wheat-Based Diets, Deficient in the Total Content of Sulfphur-Containing Amino Acids (Between 8 and 10% Below Requirement) With and Without the Additino of Either DL-Methionine Or Alimet,” http://www.novusint.com/Public/Library/en-us/PDF/Roslin%2520Final%2520Report.pdf&e=9797, Accessed January 19, 2005 The bodyweight estimation uses the final day 14 weights given in Whitehead et al.
- Aburto and Britton, “Effects of Different Levels of Vitamins A and E on the Utilization of Cholecalciferol by Broiler Chickens,” Poultry Science, 77 (1998) 570-577.
- Using the same daily feed intake from Knox et al. Slight error will be introduced into the estimation because the Knox et al. figure applies to the mean intake over the first 14 days while the Aburto and Britton study lasted for 16 days. The bodyweight is assumed to be the 16-day bodyweight given in Aburto and Britton (1998) 570-577.
- Aburto, et al., “The influence of Vitamin A on the Utilization and Amelioration of Toxicty of Cholecalciferol, 25-Hydroxycholecalciferol, and 1,25 Dihydroxycholecalciferol in Young Broiler Chickens,” Poultry Science, 77 (1998) 585-593.
- Using the same daily feed intake from Knox et al. The same room for error applies as noted in the analysis of the previous study. The bodyweight is assumed to be the 16-day bodyweight given in Aburto et al. (1988) 585-593.40.a. Since IU is a measure of physiological activity, it technically should not be used in this way to measure the more active forms of vitamin D. It is, however, used in the text to express the weight or mass of the metabolites in terms with which the reader will be familiar.
- Metz et al., “The Interaction of Dietary Vitamin A and Vitamin D Related to Skeletal Development in the Turkey Poult,” J. Nutr. 115 (1985) 929-935.
- ind, et al., “Subclinical hypervitaminosis A in rat: Measurements of bone mineral density (BMD) do not reveal adverse skeletal changes,” Chemico-Biological Interaction, 159 (2006) 73-80.
- Price, op cit. p. 334.
- Blankenship, et al., “Mecahnisms of TCDD-induced abnormalities and embryo lethality in white leghorn chickens,” Comparative Biochemistry and Physiology, Part C 136 (2003) 47-62.
- Masterjohn, Chris, “Dioxins in Animal Foods: A Case for Vegetarianism,” Published October 17, 2005. Accessed January 21, 2006.
- Berson, et al., “A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa,” Arch Opthalmol, Vol. 111 No. 6 (1993) 761-72,
- Dommisse, John, “B12: Difficulties and Obstacles in Diagnosing and Treating B12 Deficiency,” Wise Traditions 2005, November 12, 2005.
- Masterjohn, Chris, “Vitamin A: The Forgotten Bodybuilding Nutrient,” Published December 15, 2004. Accessed January 21, 2006.
- Fallon and Enig, “Vitamin A Saga,” Published March 30, 2002. Accessed January 21, 2006.
- Bastie, et al., “Cooperative action of 1a,25-dihydroxyvitaminD3 and retinoic acid in NB4 acute promyelocytic leukemia cell differentiation is transcriptionally controlled,” Experimental Cell Research, 310 (2005) 319-330.
- Greer, et al., “Vitamin A levels in patients with CF are influenced by the inflammatory response,” J Cyst Fibros Vol. 3 No. 3 (2004) 143-9.
- Russell, Robert M., “The vitamin A spectrum: from deficiency to toxicity,” Am J Clin Nutr, 71 (2000) 878-84.
- Tang, et al., “Short-term (intestinal) and long-term (postintestinal) conversion of ß-carotene to retinol in adults as assessed by a stable-isotope reference method,” Am J Clin Nutr, 78 (2003) 259-66.
- West et al., “Consequences of Revised Estimates of Carotenoid Bioefficacy for Dietary Control of Vitamin A Deficiency in Developing Countries,” J Nutr, 132 (2002) 290S-292S.
- Kardinaal, et al., “Relations between Antioxidant Vitamisn in Adipose Tissue, Plasma, and Diet,” Am J Epidemiol 141 (1995) 440-50.
- De Pee, et al., “Who has a high vitamin A intake from plant foods, but a low serum retinol concentration? Data from women in Indonesia,” Eur J Clin Nutr, Vol. 53 No. 4 (1999) 288-97.
- Bulux, et al., “Plasma response of children to short-term chronic ß-carotene supplementation,” Am J Clin Nutr, 59 (1994) 1369-75.
- Tang, et al., “Green and yellow vegetables can maintain body stores of vitamin A in Chinese children,” Am J Clin Nutr, 70 (1999) 1069-76.
- Lewis et al., “Vitamin A turnover in rats as influenced by vitamin A status,” J Nutr, Vol. 111 No. 7 (1981) 1135-44.
- Green et al., “Variation in retinol utilization rate with vitamin A status in the rat,” J Nutr Vol. 117 No. 4 (1987) 694-703.
- Rosenfeld, Louis, “Vitamine – vitamin. The early years of discovery,” Clinical Chemistry, Vol. 43 No. 4 (1997) 680-685
- “Nutrition classics. The American Journal of Hygiene, volume I, 1921: Studies on experimental rickets. VII. The relative effectiveness of cod liver oil as contrasted with butter fat for protecting the body against insufficient calcium in the presence of normal phosphorus supply. By P.G. Shipley, E.A. Park, E.V. McCollum and Nina Simmonds.” Nutr Rev, Vol. 42 No. 5 (1984) 192-4.
- Price, Weston A., op cit. p. 443.
- Razaitis, Lynn, “The Liver Files: Recipes and Lore About Our Most Important Sacred Food,” Published July 29, 2005. Accessed January 22, 2006.
- Sullivan, Krispin, “The Miracle of Vitamin D,” Published December 31, 2000. Accessed January 22, 2006.
- Garden of Life, “Olde World Icelandic Cod Liver Oil: Lemon Mint Flavor,” http://www.gardenoflifeusa.com/detail_cod_liver_sup.shtml Accessed January 22, 2006.
- Green Pastures, “Cod Liver Oil,” http://www.greenpasture.org/cod_liver_oil.php Accessed January 22, 2006.
- Brustad, et al., “Vitamin D status in a rural population of northern Norway with high fish liver consumption,” Public Health Nutrition, Vol. 7 No. 6 (2004) 783-789.
- Nieves et al., op cit. p. 816.
- Voisin, Andre, Soil, Grass and Cancer, Austin: Acres USA (2000) Chapter 8.
- Becker, Robert O. and Gary Selden, The Body Electric, New York: Morrow (1985) Chapter 8.
- Zanello and Norman, “Rapid modulation of osteoblast ion channel responses by 1a,25(OH)2-Vitamin D3 requires the presence of a functional vitamin D nuclear receptor,” PNAS, Vol. 101 No. 6 (2004) 1589-1594.
- Menaa, et al., “1,25-Dihydroxyvitamin D3 Hypersensitivity of Osteoclast Precursors from Patients with Paget’s Disease,” Journal of Bone and Mineral Research, Vol. 15 No. 2 (2000) p. 228.
- Bastie, et al., “1a, 24-Dihydroxyvitamin D3 Transrepresses Retinoic Acid Transcriptional Activity via Vitamin D Receptor in Myeloid Cells,” Molecular Endocrinology, Vol. 18 No. 11 (2004) 2685-2699.
This article appeared in Wise Traditions in Food, Farming and the Healing Arts, the quarterly magazine of the Weston A. Price Foundation, Winter 2005/Spring 2006.
🖨️ Print post

Why are there no comments? or a billion likes via facebook? This is awesome information Mr. Masterjohn! Thank you so much for all the hard work you do.
TC, let’s give him his due. It’s Doctor Masterjohn. I gladly read or listen to anything he puts out. I love his “real-world” approach to research.
Thank you for this very informative article. I wish more practitioners understood this relationship between vitamin A & D.
I’m in total awe of this research! Thank you, thank you, thank you!
Thank you for this article! This is great information but still leaves me with questions about the overall diet in which to consume cod liver oil and liver. We just have to do the best we can and keep researching. It’s tough too, when trying to feed small children, my husband,and myself on a limited budget.
To clarify, are you suggesting that a ratio of 10:1 vitamin A to vitamin D to be ideal or, as we are currently doing, would it be okay to have closer to a 1:1 ratio or even more vitamin D than vitamin A? I’m currently giving small doses of a high vitamin virgin cod liver oil supplying 1250-5000(1/4 tsp to 1 tsp)IU A and 50-300 IU D, along with 1000-3000 IU D3 from other supplements to my 3 and 7 year old boys.They are also taking 90mcg mk7 2-3 times per week. I also plan on starting my 7 month old on supplements soon. I usually take one tsp. of the cod liver oil myself.I’m going to give liver a try for another source of A. I usually take 6000-12,000 IU vitamin D3, because I’m breastfeeding and very overweight, to ensure my baby is getting enough.
Is synthetic mk4(synthesized from tobacco leaves) a good source of supplemental k2? I’m currently considering Thorne k2 and or D/k2 combo which has synthesized menatetrenone. To your knowledge is this a safe supplement for children? I think you mentioned in the Activator X article that it was believed to be physiologically identical to natural mk4. Is this correct? I’m aiming for an amount that would be normal in food up to 1mg or 1000 mcg. Less for the younger ones, more for my 7 year old with dental issues. More for my husband and me. Although, I am breastfeeding and don’t want to overdo (or underdo) the amount. I’m leaning towards mk4 being better than mk7 but would like some confirmation on type and amounts.
There’s no way they will eat butter oil and it is just too expensive for us right now. I’ve just recently been able to get them to eat pasteured butter on toast with a little jam. They do fine with cod liver oil though. We are also trying to increase natural sources through pasteured butter and ghee, and want to add pasteured raw milk, meat and eggs. We have a limited budget and ,so far, have only been able to add the better butter and ghee and we are just eating commercial eggs, whole milk, whole milk yogurt and very little meat while trying to increase fruit vegetables and replace white bread with homemade whole wheat, sprouted, sourdough. My kids won’t eat that much of the butter and I want to supplement to ensure an adequate amount in addition to continually working to improve our diet.
Let me just add that, I know you’re not my medical doctor and can’t be responsible for other’s health decisions. I’m just looking for an educated opinion to consider,as a part of my research. I would truly appreciate it if you could give your opinion on the questions I have asked.
Thank you so much, again, for this article and all the others!
Aimee J.
Aimee, I wish someone had answered you here! Did you ever get these questions answered for yourself? If so, what did you find out?
He is saying that vitamin D is like a switch to change vitamin A from causing bone density loss in proportion to how much you take, to the more A you have, the better for bone density. It’s not even the ratio, but having a sufficient amount. However, only sunlight or ridiculous amounts of supplementation can boost D levels into the optimal levels. I have grass-fed butter (Kerry Gold, although they do admit to using ~3% GMO’s), and will from here on out, avoid the Green Pastures products, which are shockingly blase about rancidity (read info from Dr. Kaayla Daniel on this). There’s a St. Clement’s which seems to be a good one.
Regarding your comment on fermented cod liver oil, WAPF has responded to this here:
https://www.westonaprice.org/health-topics/update-on-fermented-cod-liver-oil/
Also here:
https://www.westonaprice.org/health-topics/cod-liver-oil/hook-line-and-thinker-adding-context-to-kaayla-daniels-report-on-fermented-cod-liver-oil/
And several other links here:
https://www.westonaprice.org/cod-liver-oil/
Fermented cod liver oil showed the best stability 6 weeks after opening when compared to several other brands.
I myself trust Green Pasture brand and don’t consider it more rancid than other brands. All cod liver oils can go rancid, depending on storage conditions and are not necessarily better in that regard.
I’m looking for WAPF info on vitamin E complex. This article came up. Nothing on vitamin E though. No animal fat is really high in E – or could it be in the brain & sex organs? No listing on those, so it must be so. Highest is soy oil, but today’s oils are all overrefined & overfiltered. Food fascism at work again.
Well this might explain why taking high dose vitamin D is a bad idea. Why initially I felt good taking vitamin D and subsequently felt no positive benefit.